Consider the equilibrium reaction shown below. B2(g) 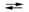 2B(g)
2B(g)
If the rate constants are: kfwd = 7.00 * 10¯5 s¯1 and krev = 2.00 * 10¯5 L mol¯1 s¯1, what is the value of Kc under these conditions?
Definitions:
Builds Trust
The process of establishing credibility and reliability with others, crucial for fostering healthy relationships and teamwork.
Role Model
An individual admired for their behaviors, qualities, or achievements, serving as an example for others to emulate.
Zand's Model
A theoretical framework focusing on trust within organizations, proposing that trust influences problem-solving and information processing in teams.
Trust
The reliance on the integrity, strength, and ability of a person or entity, which forms the basis of relationships in social, economic, and business contexts.
Q3: The solubility of lead(II) chloride is 0.45
Q14: Given this equilibrium constant data at
Q19: A solution is prepared by adding 0.10
Q24: Select the correct name for the following
Q28: Raoult's Law relates the vapor pressure of
Q73: Chlorine atoms act as heterogeneous catalysts in
Q77: For a gas-phase equilibrium, a change in
Q83: Consider the reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7799/.jpg" alt="
Q87: Select the correct name for the following
Q93: Hydrogen sulfide will react with water as