The equilibrium constant, Kp, for the reaction 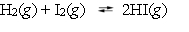 is 55.2 at 425 C. A rigid cylinder at that temperature contains 0.127 atm of hydrogen, 0.134 atm of iodine, and 1.055 atm of hydrogen iodide. Is the system at equilibrium?
is 55.2 at 425 C. A rigid cylinder at that temperature contains 0.127 atm of hydrogen, 0.134 atm of iodine, and 1.055 atm of hydrogen iodide. Is the system at equilibrium?
Definitions:
Tailgating
An unauthorized person following someone with authorized access through a secure entryway, or a safety issue related to driving too closely behind another vehicle.
Unintentional Threat
A security risk that arises without malicious intent, often due to negligence or lack of awareness.
Cybercrime
Illegal activities executed on the Internet.
Interconnected
Describes systems or devices that are connected to each other through various communication channels, allowing for interaction and data exchange.
Q3: From the following list of aqueous solutions
Q13: Which, if any, of the following aqueous
Q14: Amygdalin (Laetrile) was once touted for its
Q14: An increase in temperature increases the reaction
Q27: An aqueous solution is prepared by dissolving
Q35: Magnesium carbonate dissociates to magnesium oxide
Q55: The equilibrium constant, K<sub>p</sub>, for the
Q67: List five important intermolecular forces that operate
Q73: The concentration of iodine in sea water
Q96: A sample of octane in equilibrium