Consider the reversible reaction: 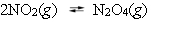 If the concentrations of both NO2 and N2O4 are 0.016 mol L¯1, what is the value of Qc?
If the concentrations of both NO2 and N2O4 are 0.016 mol L¯1, what is the value of Qc?
Definitions:
Internal Control-Integrated Framework
A conceptual framework developed by the Committee of Sponsoring Organizations (COSO) to help businesses establish, assess, and enhance their internal control systems.
Analyzing
The process of examining data or information to understand its components, relationships, and implications, often for decision-making purposes.
Evaluating
The process of assessing or examining something to determine its value, effectiveness, or condition, often for decision-making purposes.
Bank Reconciliation
The process of matching the balances in an entity's accounting records for a cash account to the corresponding information on a bank statement, to identify discrepancies and reconcile differences.
Q19: Calculate <span class="ql-formula" data-value="\Delta"><span class="katex"><span
Q20: A red glaze on porcelain can be
Q27: Which one of the following groups does
Q35: An elementary reaction is a simple, one-step
Q47: A 0.100 m K<sub>2</sub>SO<sub>4</sub> solution has
Q55: Use the following information to calculate the
Q55: The chemical symbol for potassium is:<br>A) P<br>B)
Q81: A lab technician adds 0.015 mol of
Q86: elements are strongly acidic.
Q98: What are the approximate carbon:hydrogen mass ratios