The equilibrium constant Kc for the reaction 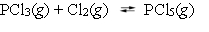 is 49 at 230 C. If 0.70 mol of PCl3 is added to 0.70 mol of Cl2 in a 1.00-L reaction vessel at 230 C, what is the concentration of PCl3 when equilibrium has been established?
is 49 at 230 C. If 0.70 mol of PCl3 is added to 0.70 mol of Cl2 in a 1.00-L reaction vessel at 230 C, what is the concentration of PCl3 when equilibrium has been established?
Definitions:
Scrap Disposal
The process of getting rid of waste materials that are produced during manufacturing or consumption.
Emissions Fee
A charge levied on the production, use, or release of pollutants, aiming to reduce environmental harm by incentivizing cleaner practices.
Optimum Level
The most advantageous condition or degree of something where the optimal balance or efficiency is achieved.
Fixed Annual Fee
A fixed annual fee is a set amount charged yearly for access to a product or service, unrelated to usage levels.
Q8: Which of the following elements could be
Q9: You are studying the rate of
Q20: A reaction has a positive value
Q23: Octane is a component of fuel used
Q32: A 0.89% (w/v) sodium chloride solution is
Q52: Benzaldehyde (? = 106.1 g/mol), also
Q72: Which of the following is a metalloid?<br>A)
Q76: Predict the products for the following
Q89: Consider this reaction: 8A(g) + 5B(g)
Q106: Select the correct name for this compound.