The reaction of nitric oxide to form dinitrogen oxide and nitrogen dioxide is exothermic. 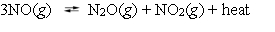 What effect will be seen if the temperature of the system at equilibrium is raised by 25 C?
What effect will be seen if the temperature of the system at equilibrium is raised by 25 C?
Definitions:
High Insulin
A condition characterized by elevated levels of insulin in the blood, often associated with type 2 diabetes.
Americans
Citizens or inhabitants of the United States of America, or pertaining to aspects of American culture, society, or national identity.
Blood Sugar
The concentration of glucose present in the blood, important for maintaining energy levels.
Ventromedial Hypothalamus
A region of the brain that plays a critical role in satiety and the regulation of hunger.
Q6: Which of the following sets of
Q13: In the electrolyte of an electrochemical cell,
Q23: Select the correct name for this compound.
Q50: Cyclopropane is converted to propene in a
Q63: Calculate the solubility of silver phosphate, Ag<sub>3</sub>PO<sub>4</sub>,
Q65: Which of the following statements concerning a
Q68: Which of the following has the highest
Q86: The meniscus of mercury in a glass
Q94: Which of the following oxides is most
Q98: What are the approximate carbon:hydrogen mass ratios