The following reaction is at equilibrium in a closed container. 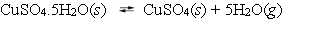 Which, if any, of the following actions will lead to an increase in the pressure of H2O present at equilibrium?
Which, if any, of the following actions will lead to an increase in the pressure of H2O present at equilibrium?
Definitions:
Deposit Expansion Multiplier
The ratio that quantifies the potential increase in total bank deposits that can result from an initial deposit, through the process of banks lending and creating more deposits.
Reserve Ratio
The fraction of deposits that banks are required to keep on hand and not lend out, as mandated by banking regulations.
Open Market Operations
The buying and selling of government securities in the open market by a central bank to control the supply of money.
Money Supply
The overall amount of available money in an economy, which encompasses cash, coins, and the balances in checking and savings accounts, at a certain moment in time.
Q1: The substance HClO<sub>4</sub> is considered<br>A) a weak
Q8: For a solution equilibrium, a change in
Q15: Calculate the vapor pressure of a
Q26: The coordination number of sodium and chloride
Q41: Calculate the solubility of magnesium sulfate, MgSO<sub>4</sub>,
Q61: Chlorine dioxide is a strong oxidizer that
Q94: Which of the following oxides is most
Q95: Which of the following substances is produced
Q100: Consider the equilibrium reaction shown below. B<sub>2</sub>(g)
Q107: Which element forms compounds which are used