Briefly outline the key arguments in the collision theory of reaction rates for the elementary reaction 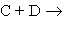 products. Show that this theory predicts a second-order rate law, and how it predicts the form of the rate constant k.
products. Show that this theory predicts a second-order rate law, and how it predicts the form of the rate constant k.
Definitions:
Equilibrium Price
The price at which the quantity of a product demanded by consumers equals the quantity supplied by producers.
Consumer Surplus
The divergence between the total payment consumers are willing to make for a product or service and what they really pay.
Demand Increase
A situation where consumers are willing and able to purchase more of a product or service at each price level, often caused by changes in tastes, income, or prices of related goods.
Consumer Surplus
The separation in monetary terms between what consumers are willing to pay for a good or service and their final payment amount.
Q2: Potassium is a strong reducing agent.
Q3: From the following list of aqueous solutions
Q12: Predict the products for the reaction
Q20: When a catalyst is added to a
Q29: Draw a fully labeled phase diagram (P
Q63: When identical particles pack in a simple
Q69: Pentane, C<sub>5</sub>H<sub>12</sub>, boils at 35 <span
Q69: Sulfuryl chloride, SO<sub>2</sub>Cl<sub>2</sub>(g), decomposes at high temperature
Q71: The reaction system <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7799/.jpg" alt="The reaction
Q83: In the collision theory of reaction rates,