Consider the following mechanism for the oxidation of bromide ions by hydrogen peroxide in aqueous acid solution. 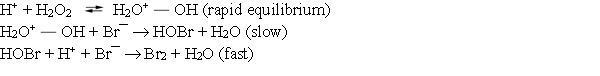 What is the overall reaction equation for this process?
What is the overall reaction equation for this process?
Definitions:
Fencing
The sport of fighting with swords, particularly foils, épées, or sabres, according to set rules; also refers to a barrier or structure serving as a boundary or means of protection.
Mowing
The process of cutting down grass or other vegetation to maintain land or lawn appearance and health.
Special Warranty Deed
A type of deed in which the seller guarantees the title only against defects arising during their ownership and not against defects existing before that time.
Warrants
Warrants are financial instruments issued by companies that give the holder the right to purchase company stock at a specific price before a certain date.
Q2: In a spontaneous process, the entropy of
Q5: Saccharin, one of the first non-nutritive sweeteners
Q19: A phosphate buffer (H<sub>2</sub>PO<sub>4</sub>¯/HPO<sub>4</sub><sup>2</sup>¯) has a pH
Q19: Which one, if any, of the following
Q38: Write the expressions for K<sub>c</sub> and K<sub>p</sub>
Q50: Cyclopropane is converted to propene in a
Q55: Potassium fluoride is used for frosting glass.
Q62: Which one of the following formulas of
Q68: Condensation polymers are formed by free radical
Q78: The greater the energy of activation, E<sub>a</sub>,