When magnesium reacts with hydrochloric acid, aqueous magnesium chloride and hydrogen gas are produced.Suppose 0.120 g Mg react with the HCl in a 75.000 g acidic solution contained in a calorimeter.The temperature increases by 6.03 C.Calculate the Hrxn of Mg with HCl in kJ/mol.Assume the specific heats of the solution and of the empty calorimeter are 4.18 J/g . C) and 64.4 J/ C, respectively.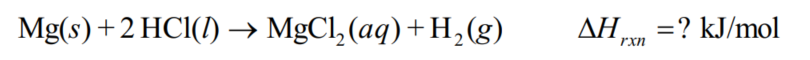
Definitions:
Schizophrenia
A mental disorder characterized by disturbances in thought, perception, and behavior, including delusions, hallucinations, and disorganized speech.
Panic Disorder
A type of anxiety disorder characterized by sudden and recurrent panic attacks that involve overwhelming fear and physical symptoms.
Social Phobia
A chronic mental health condition characterized by an intense fear of social situations, leading to avoidance and distress.
Major Depressive Disorder
A mental health condition characterized by persistent feelings of sadness, loss of interest in activities, and various emotional and physical problems.
Q43: A solution is made by dissolving
Q57: Quicklime, CaO (56.08 g/mol), and water can
Q59: The van der Waals constants for NO<sub>2</sub>
Q93: The reaction of nitrogen with oxygen
Q97: Silver metal reacts with nitric acid to
Q123: Hemlock contains a number of toxins, one
Q141: Which of the following gases could most
Q151: A sample of propane gas is contained
Q191: If a chemical reaction causes the temperature
Q192: Ethanol (CH<sub>3</sub>CH<sub>2</sub>OH) has been suggested as