Based on the information in the table of standard reduction potentials below, what is the standard cell potential for an electrochemical cell that has iron Fe) and magnesium Mg) electrodes immersed in 1M Fe3+and Mg2+solutions? Also, identify the cathode. 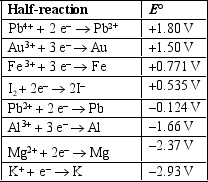
Definitions:
Management
The process of dealing with or controlling things or people, often within an organizational setting, including planning, decision-making, and overseeing operations.
Productivity
A measure of the efficiency of production, often expressed as the ratio of outputs to inputs in the production process.
Nonunionized Companies
Businesses where the workforce is not represented by a labor union, and where bargaining and negotiations are conducted individually rather than collectively.
Unionized Companies
Companies where a workforce is formally represented by a labor union to negotiate wages, benefits, and working conditions.
Q6: If the temperature of an endothermic reaction
Q10: Consider the equilibrium CL<sub>2</sub> (g) + 2
Q38: In the unit cell of sphalerite,<br>A)all the
Q40: Delocalized bonding in aromatic hydrocarbons is possible
Q58: Which of the following refers to an
Q65: K<sub>sp</sub> for lead iodide is 8.5 *10<sup>-9</sup>
Q65: Iron (Fe) crystallizes as a body-centered unit
Q72: A monosaccharide an empirical formula of<br>A)CH<sub>2</sub>O.<br>B)C<sub>6</sub>H<sub>12</sub>O<sub>6</sub>.<br>C)C<sub>12</sub>H<sub>2</sub>2O11.<br>D)C<sub>2</sub>H<sub>4</sub>O<sub>2</sub>.<br>E)C<sub>3</sub>H<sub>6</sub>O<sub>3</sub>.
Q89: Which of the following is characteristic
Q120: Neuron cells generate electrical signals by concentration