Suppose the reaction 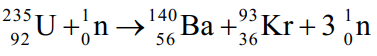 produces 1.664 *1010 kJ/mol of energy.
produces 1.664 *1010 kJ/mol of energy.
Calculate the change in mass in grams that occurs when one mole of U-235 reacts with one mole of neutrons.E = mc2, where c = 2.998 *108 m/s; 1 kg = 6.0221415 *1026 amu; 1 J = 1kg · m2/s2.
Definitions:
Critical Theory
A philosophical approach that seeks to understand and critique society and culture, advocating for societal changes.
Political Economy
An interdisciplinary field that examines the relationship between politics and economics, focusing on the ways in which political institutions, the political environment, and the economic system influence each other.
Karl Marx
A 19th-century philosopher, economist, and political theorist, whose ideas about class struggle and capitalism laid the foundation for Marxist theory.
Concrete Manifestations
Tangible or visible expressions of ideas, concepts, or phenomena that can be observed and measured in the real world.
Q17: Write a balanced chemical equation using skeleton
Q19: What is the most important limitation of
Q37: Which of the following metal ions can
Q47: In its ring structure, which functional groups
Q74: The reaction, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6562/.jpg" alt=" The
Q78: Radioactive 90Sr can substitute for _ in
Q100: Aspartame exists as a zwitterion at physiological
Q105: What is the correct name for the
Q110: A significant positive correlation is found between
Q112: All of the following are disadvantages of