Consider a voltaic cell that corresponds to the following reaction: Cu(s) + 2Ag+(aq) Cu2+(aq) + Ag(s) If this reaction takes place in the electrochemical cell shown in the figure, which of the following statements is incorrect? 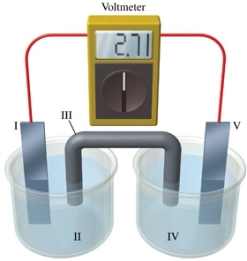
Definitions:
Serotonin Activity
Refers to the actions and effects of serotonin, a key neurotransmitter, in the brain which impacts mood, feelings of well-being, and happiness.
Aerobic Exercise
Physical activity that improves heart and lung function, increases endurance, and often involves rhythmic, continuous movement.
Blood Pressure
The force exerted by circulating blood on the walls of blood vessels, considered vital for human survival and an important indicator of health.
Problem-Focused Coping
A strategy wherein an individual tackles a problem head-on by addressing the issue directly to mitigate or eliminate its impact.
Q5: What ions, atoms, or molecules (in addition
Q11: In which substance does chlorine have an
Q12: Standardized services are provided by companies that
Q25: Examine the following model of a carbohydrate
Q28: If the base sequence in a portion
Q39: In an effort to avoid redundant research,
Q41: Extrapolation from present data is an effective
Q60: The images represent a solution of NaNO<sub>3</sub>,
Q78: Consider the reaction: CrO<sub>4</sub><sup>2</sup><sup>-</sup>(aq) + HSO<sub>3</sub>-(aq)
Q90: Determine the moles of solute particles in