A carbene in the singlet state is typically portrayed as sp2 hybridized, with a vacant p -orbital and a pair of nonbonding electrons residing in one sp2 hybrid orbital. Using molecular orbital theory, describe how a neighboring 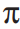 -bond can act as a nucleophile with the carbene in the formation of a cyclopropyl ring.
-bond can act as a nucleophile with the carbene in the formation of a cyclopropyl ring.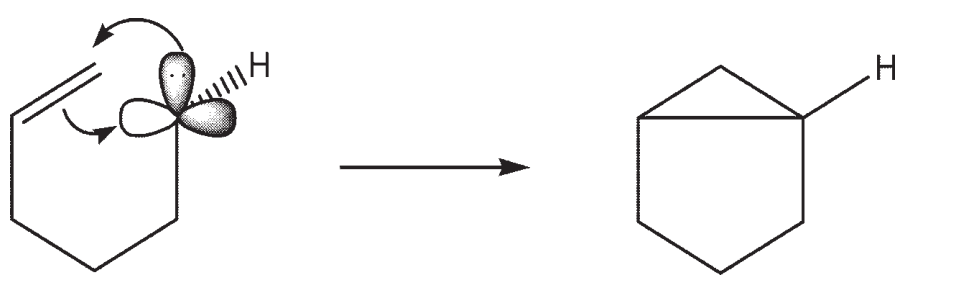
Definitions:
Total Energy Expenditure
The amount of calories burned by an individual through basal metabolic rate, physical activity, and thermic effect of food over a given period.
SMART Goal
A framework for setting specific, measurable, achievable, relevant, and time-bound objectives.
Fasting Blood Glucose
A test that measures the levels of glucose in the blood after an individual has not eaten for a certain period, used to diagnose diabetes.
High-risk Waist Circumference
A measurement indicative of a higher risk of developing obesity-related health conditions, usually based on guidelines that specify cutoff points for men and women.
Q20: Draw a mechanism for the hydration of
Q23: A machine dispenses a liquid drug into
Q34: Which of the following residues does not
Q70: Draw a mechanism for the following transformation.Include
Q81: A random sampling of sixty pitchers from
Q88: In a Gallup poll of 557 randomly
Q121: The mean resting pulse rate for
Q125: Write the claim that is suggested by
Q182: x1 = 59, n1 = 117 and
Q183: <span class="ql-formula" data-value="\begin{array} { c | c