Consider the reaction N2(g) + O2(g) → 2NO(g) . The molecular image represents a mixture of N2(g) and O2(g) just before reaction occurs. What is the limiting reactant, and how much of the excess reactant remains after the reaction is complete? The image contains 2 N2 molecules and 4 O2 molecules. 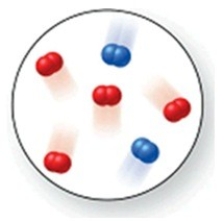
Definitions:
Guided Participation
A teaching method where an experienced person helps a learner by gradually adjusting the level of support as the learner's competence increases.
Vygotsky
A theorist known for emphasizing the social and cultural context in the development of cognition, highlighting the importance of language and social interaction.
Guided Participation
A learning process in which an experienced person helps someone less experienced by engaging in shared activities and providing insights.
Cultural Knowledge
Knowledge about the practices, beliefs, values, norms, and artifacts of a group that are passed down from generation to generation.
Q10: Aluminum reacts with oxygen according to the
Q15: Which of the following statements regarding ionic
Q20: Which of the following is the correct
Q33: The abbreviated electron configuration [Kr] applies to
Q45: The formula for calcium sulfide is:<br>A)Ca<sub>2</sub>S<br>B)CaS<sub>2</sub><br>C)CaS<br>D)Ca<sub>2</sub>S<sub>2</sub><br>E)Ca<sub>2</sub>S<sub>3</sub>
Q68: Calculate the molar mass of C<sub>3</sub>H<sub>6</sub>Cl<sub>2</sub>.<br>A)155.08 g/mol<br>B)77.54
Q82: Which of the following statements regarding electromagnetic
Q84: 3.00 moles of NaOH (sodium hydroxide)are needed
Q86: The images in the figure represent aqueous
Q113: When solid ammonium carbonate is heated, it