Consider the following specific heats of metals. 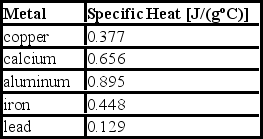 If the same amount of heat is added to 25.0 g of each of these metals, all at the same initial temperature, which metal will have the highest final temperature?
If the same amount of heat is added to 25.0 g of each of these metals, all at the same initial temperature, which metal will have the highest final temperature?
Definitions:
Inconsistencies
Occurrences of being in disagreement or not being compatible with previously established facts, logic, or claims.
Technology Issues
Problems or challenges associated with information technology, including hardware malfunctions, software bugs, and cybersecurity vulnerabilities.
Writing Center
A resource available in many educational institutions providing students with assistance in writing tasks, including tutoring, editing, and feedback.
Revising
The process of reviewing, altering, and amending content or documents with the aim of improving them.
Q11: When completing the orbital diagram for the
Q46: The images in the figure represent aqueous
Q58: When aqueous solutions of potassium chloride and
Q68: Propane burns in air according to the
Q69: Which of the following is the correct
Q75: When mixed, solutions of aluminum nitrate, Al(NO<sub>3</sub>)<sub>3</sub>,
Q83: Calculate the number of moles and the
Q98: Which of the following is not a
Q117: Predict the molecular shape and give the
Q126: How much heat must be added to