Consider the reaction N2(g) + O2(g) → 2NO(g) . The molecular image represents a mixture of N2(g) and O2(g) just before reaction occurs. What is the limiting reactant, and how much of the excess reactant remains after the reaction is complete? The image contains 2 N2 molecules and 4 O2 molecules. 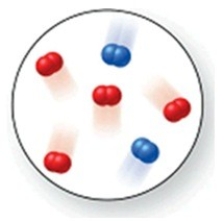
Definitions:
Temporal
Relating to time or the sequential order in which events occur.
Casual
An informal, relaxed approach or style, often applied to dress codes, conversation, or workplace environment.
Intuitive Judgments
Decision making that is based on instinctive feelings rather than conscious reasoning or detailed analysis.
Devil's Advocate
An individual who puts forward a controversial view with the aim of sparking discussion or assessing the robustness of counterarguments.
Q4: Which of the following observations does not
Q21: The formula for the bromate ion is
Q23: Calculate the molar mass of O<sub>2</sub> in
Q46: Write the element symbol for the element
Q72: Consider the following reaction: 3NO<sub>2</sub>(g)+ H<sub>2</sub>O(l)→ 2HNO<sub>3</sub>(l)+
Q73: Which of the following combinations of formula
Q97: The color of visible light with the
Q105: Predict the molecular shape and give the
Q107: When potassium metal is exposed to air,
Q111: A piece of magnesium metal gradually forms