The wave functions of some molecules are a combination of wave functions with different values of the orbital quantum number 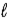 . The wave function of PF5 combines s, p and d states in an sp3d hybrid orbital. We would expect such an overlap of wave functions in individual molecules to represent
. The wave function of PF5 combines s, p and d states in an sp3d hybrid orbital. We would expect such an overlap of wave functions in individual molecules to represent
Definitions:
Murmur Auscultated
The sound heard through a stethoscope that indicates the turbulent flow of blood in the heart or great vessels.
Venous Hum
A continuous, low-pitched sound heard over the major veins, particularly in the neck, due to turbulent blood flow.
Murmur
Abnormal heart sounds during a heartbeat cycle, which can indicate various heart conditions.
Carotid Artery
A major blood vessel in the neck that supplies blood to the brain, neck, and face.
Q5: The graph below shows the value of
Q6: A contact lens is made of plastic
Q16: In order to jump off the floor,
Q19: A narrow slit is illuminated with sodium
Q26: A concave mirror has a radius of
Q32: Potassium Iodide (chemical formula: KI) has a
Q39: The speed of a particle moving in
Q41: Starting from one oasis, a camel walks
Q50: Determine the rms voltage drop across the
Q107: A 2000-kg sailboat experiences an eastward force