In the subshell of the Li2+ ion with orbital quantum number 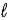 , the allowed values of the magnetic quantum number
, the allowed values of the magnetic quantum number 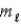 are
are
Definitions:
Reinforcements
Stimuli or outcomes that strengthen the probability of a behavior's occurrence, either by presenting a positive stimulus or removing a negative one.
Humans
A species of highly intelligent primates known scientifically as Homo sapiens, characterized by bipedalism, complex language use, and advanced social structures.
Excessive Consumption
The act of consuming goods or resources in quantities greater than those considered normal or sustainable.
Alcohol
A psychoactive substance found in beverages such as beer, wine, and spirits, known for its intoxicating effects.
Q4: A 0.50 kg mass attached to the
Q6: Name at least one conservation law that
Q13: A stunt pilot performs a circular dive
Q16: A molecule makes a transition from the
Q20: When at rest, a spacecraft has the
Q29: The wave function for a particle confined
Q34: A car travels in a flat circle
Q39: The Fermi temperature of copper is 80
Q73: At t = 0, a particle leaves
Q96: The horizontal surface on which the block