The radial portion of the de Broglie wavefunction for an electron in the ground state of the hydrogen atom is Ψ1s(r) = 1/( 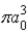 ) 1/2 exp(−r/a0) where a0 is the Bohr radius. The probability of finding the electron is
) 1/2 exp(−r/a0) where a0 is the Bohr radius. The probability of finding the electron is
Definitions:
Infiltration
The process by which substances or cells pass through tissue, often referring to the spread of cancer cells or the delivery of fluids/medication.
Temperature Control
The process of regulating the temperature of a system or environment through natural or artificial means.
Pathogens
Microorganisms or viruses that can cause disease.
Soft Nail
A condition characterized by nails that are easily bent, broken, or split, often indicating underlying health issues or nutrient deficiencies.
Q14: If the quark content of the proton,
Q14: Light reflected off a plate-glass window (n
Q23: At an instant when a 4.0-kg object
Q33: Refer to Exhibit 3-4. The total displacement
Q41: In a location where the train tracks
Q44: The figure shows two point sources of
Q45: In the figure, if the tension in
Q50: What value of Z (atomic number) and
Q67: A 1.5-kg object moving along the x
Q71: If the scalar (dot) product of two