Hydrogen atom: The normalized wave function for a hydrogen atom in the 1s state is given by ψ(r) = 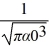 e-r/α0 where α0 is the Bohr radius, which is equal to 5.29 × 10-11 m. What is the probability of finding the electron at a distance greater than 7.8 α0 from the proton?
e-r/α0 where α0 is the Bohr radius, which is equal to 5.29 × 10-11 m. What is the probability of finding the electron at a distance greater than 7.8 α0 from the proton?
Definitions:
Put Option
A financial contract giving the buyer the right, but not the obligation, to sell an underlying asset or security at a specified price within a defined time period.
Risk Profile
An individual's or entity's willingness to take risks, as well as the potential financial losses they can afford.
Price Fluctuations
Price fluctuations refer to variations in the selling price of goods and services over a period, influenced by market conditions.
Borrowing Costs
Expenses incurred by an entity for borrowing funds, including interest, arrangement fees, and other related costs.
Q10: Power: The work performed as a function
Q10: Multielectron atoms: An atom has completely filled
Q16: Momentum: In the lab, a relativistic proton
Q19: Molecular rotation: Estimate the rotational energy (in
Q20: Banked curves: A 600-kg car traveling at
Q27: Total energy: A proton in a certain
Q28: Refraction at a curved surface: A fish
Q32: Particle in a box: A particle is
Q94: Work-energy theorem: A ball is thrown upward
Q99: General questions: A ball is tossed vertically