Hydrogen atom: The normalized wave function for a hydrogen atom in the 1s state is given by ψ(r) = 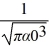 e-r/α0 where α0 is the Bohr radius, which is equal to 5.29 × 10-11 m. What is the probability of finding the electron at a distance greater than 7.8 α0 from the proton?
e-r/α0 where α0 is the Bohr radius, which is equal to 5.29 × 10-11 m. What is the probability of finding the electron at a distance greater than 7.8 α0 from the proton?
Definitions:
Education
The process of facilitating learning, or the acquisition of knowledge, skills, values, beliefs, and habits through various means.
Finance
The management of large amounts of money, especially by governments or large companies.
Ability-To-Pay Principle
The idea that those who have greater income (or wealth) should pay a greater proportion of it as taxes than those who have less income (or wealth).
Public Goods
Goods that are non-excludable and non-rivalrous, meaning they are accessible to all without diminishing availability to others, such as public parks or national defense.
Q4: Molecular rotation: When a certain diatomic molecule
Q8: Kinetic energy: In a certain particle accelerator,
Q16: Uncertainty principle using ΔEΔt ≥ ħ/2: A
Q17: Quarks: Composite particles that are composed of
Q21: Eyes: What is the uncorrected near point
Q24: Particles: Which of the following particles are
Q35: Uniform circular motion: Point P in the
Q49: Friction: A pickup truck is moving at
Q62: Air resistance: A 30.0-kg object experiences a
Q71: Blackbody radiation: A perfectly black body at