Phase changes: A substance has a melting point of 20°C and a heat of fusion of 3.9 × 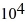 J/kg. The boiling point is
J/kg. The boiling point is 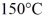 and the heat of vaporization is
and the heat of vaporization is 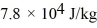 at a pressure of 1.0 atm. The specific heats for the solid, liquid, and gaseous phases are 600 J/(kg ∙ K) , 1000 J/(kg∙K) , and 400 J/(kg ∙ K) , respectively. The quantity of heat required to raise the temperature of
at a pressure of 1.0 atm. The specific heats for the solid, liquid, and gaseous phases are 600 J/(kg ∙ K) , 1000 J/(kg∙K) , and 400 J/(kg ∙ K) , respectively. The quantity of heat required to raise the temperature of 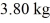 of the substance from
of the substance from 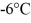 to
to 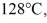 at a pressure of 1.0 atm, is closest to
at a pressure of 1.0 atm, is closest to
Definitions:
Units-Of-Production
A strategy for depreciation which assigns the cost of a property over its operational life, based on the volume of output it yields.
Double-Declining-Balance
An accelerated method of depreciation that doubles the regular depreciation rate, allowing for faster depreciation of assets in the early years.
Straight-Line
A method of calculating depreciation or amortization by dividing the difference between an asset's cost and its salvage value by the number of years it is expected to be used.
Depreciation Expense
The allocation of the cost of a tangible asset over its useful life, reflecting wear and tear, deterioration, or obsolescence.
Q3: Resistivity: As current flows through a uniform
Q8: Buoyancy: A cup of water containing an
Q16: Scalar (dot) product: If <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8274/.jpg" alt="Scalar
Q30: Energy in SHM: A 0.025-kg block on
Q34: Phase changes: A substance has a melting
Q34: Weight and g: The gravitational acceleration on
Q39: Coulomb's law: In the figure, all the
Q93: Ideal gas law: A sealed 26- <img
Q101: Interference: Two stereo speakers mounted 4.52 m
Q108: Conduction of heat: An architect is interested