Multiple Choice
Molar heat capacities: A compression, at a constant pressure of 190 kPa, is performed on 5.0 moles of an ideal monatomic gas. The compression reduces the volume of the gas from 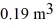 to
to 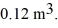 The ideal gas constant is R = 8.314 J/mol ∙ K. The work done by the gas is closest to
The ideal gas constant is R = 8.314 J/mol ∙ K. The work done by the gas is closest to
Recognize the different therapy approaches towards ideal individuals and healthy psychological functioning.
Analyze the role of conditions of worth in personal development according to different therapies.
Describe the characteristics and effectiveness of motivational interviewing.
Understand the impact of therapist's empathy, genuineness, and positive regard on therapy outcomes.
Definitions:
Related Questions
Q3: All OBD-II vehicles use what type of
Q5: Electric field and potential : If the
Q5: An oil filter should be hot drained
Q18: Satellites: The moons of Mars, Phobos (Fear)
Q34: Buoyancy: A 6.1-kg solid sphere, made of
Q45: Type of thermodynamic processes: The figure shows
Q46: Unit vectors: What is the magnitude of
Q54: Potential energy of point-charges: An alpha particle
Q59: Electric field of a single point-charge: A
Q86: Standing sound waves: An air column, open