The figure shows the pV diagram for a certain thermodynamic process. In this process, 1500 J of heat flows into a system, and at the same time the system expands against a constant external pressure of 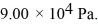 If the volume of the system increases from
If the volume of the system increases from 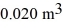 to
to 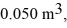 calculate the change in internal (thermal) energy of the system. If the internal (thermal) energy change is nonzero, be sure to indicate whether this energy change is positive or negative.
calculate the change in internal (thermal) energy of the system. If the internal (thermal) energy change is nonzero, be sure to indicate whether this energy change is positive or negative. 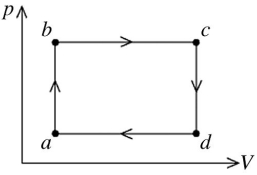
Definitions:
Intangibility
A characteristic of services that makes them unable to be seen, touched, tasted, felt, or measured in the same manner as physical goods.
Inseparability
A characteristic of service marketing that indicates the simultaneous production and consumption of a service, implying that services cannot be separated from their providers.
Services
Intangible and non-physical goods provided to consumers, including activities, benefits, or satisfactions offered for sale.
Service Continuum
Describes the range of offered services between pure tangible goods and pure services, showing how most products are a combination of both.
Q2: Potential due to point-charges: A +4.0 μC-point
Q2: What are LED, LCD, VTF, and CRT
Q8: Vector (cross) product: If the magnitude of
Q9: A keyless remote control stops working. Technician
Q17: Molecular speeds: A sample of an ideal
Q20: Coulomb's law: A + 7.00 μC point
Q42: Temperature scales: (a) Internal human body temperature
Q51: Scalar (dot) product: Two boys searching for
Q76: Sound level: An enclosed chamber with sound
Q85: Ideal gas law: How many moles of