An analytical chemist wanted to use electrolysis to determine the number of moles of cupric ions in a given volume of solution. The solution was partitioned into n = 30 portions of 0.2 milliliter each. Each of the n = 30 portions was tested. The average number of moles of cupric ions for the n = 30 portions was found to be 0.185 mole; the standard deviation was 0.015 mole. Calculate the following intervals: 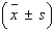 = ______________
= ______________ 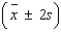 = ______________
= ______________ 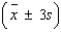 = ______________
= ______________
Describe the distribution of the measurements for the n = 30 portions of the solution using Tchebysheff's Theorem.
________________________________________________________
Describe the distribution of the measurements for the n = 30 portions of the solution using the Empirical Rule.
________________________________________________________
Suppose the chemist had used only n = 5 portions of the solution for the experiment and obtained the readings 0.18, 0.21, 0.20, 0.22, and 0.18. Would the Empirical Rule be suitable for describing the n = 5 measurements?
______________
Explain.
________________________________________________________
Definitions:
Sit-in
A form of protest where participants occupy a space, refusing to leave until their demands are met or to draw attention to an issue.
Black Is Beautiful
A cultural movement that emerged in the 1960s in the United States, celebrating African American culture and aesthetics, challenging prevailing beauty standards.
Military And Economic Aid
Assistance provided by one nation to another in the form of defense equipment, military training, or financial support to bolster the recipient's economy.
North Vietnam
The communist-led region and government that controlled the northern part of Vietnam from 1954 until the country was unified in 1976.
Q6: Define three categories of analytics.
Q8: A firm that successfully pursues a steeper-than-industry-average
Q8: The number of traffic accidents per day
Q18: The manager of a movie rental store
Q52: Explain the three requirements of data integrity.
Q65: State the three-fold idea behind simulation.
Q68: A politician who is running for the
Q84: A table, formula, or graph that shows
Q87: Twenty-eight applicants interested in working for the
Q121: In a time study, conducted at a