An analytical chemist wanted to use electrolysis to determine the number of moles of cupric ions in a given volume of solution. The solution was partitioned into n = 30 portions of 0.2 milliliter each. Each of the n = 30 portions was tested. The average number of moles of cupric ions for the n = 30 portions was found to be 0.185 mole; the standard deviation was 0.015 mole. Calculate the following intervals: 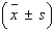 = ______________
= ______________ 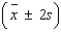 = ______________
= ______________ 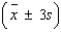 = ______________
= ______________
Describe the distribution of the measurements for the n = 30 portions of the solution using Tchebysheff's Theorem.
________________________________________________________
Describe the distribution of the measurements for the n = 30 portions of the solution using the Empirical Rule.
________________________________________________________
Suppose the chemist had used only n = 5 portions of the solution for the experiment and obtained the readings 0.18, 0.21, 0.20, 0.22, and 0.18. Would the Empirical Rule be suitable for describing the n = 5 measurements?
______________
Explain.
________________________________________________________
Definitions:
Plagiarism
The act of using someone else's work or ideas without proper acknowledgment, presenting them as one's own.
Electronic Data
Information that has been processed, stored, and transmitted in digital form, making it accessible through electronic devices.
Print Resources
Materials in printed form such as books, magazines, and newspapers used for research or information.
Direct Quotations
Direct quotations are the exact words taken from a source and incorporated into a new document, usually with proper attribution to the original speaker or writer.
Q9: The Poisson random variable is the number
Q13: The _ method is a simulation technique
Q39: A metal works fabricator is about to
Q52: The mean is one of the most
Q86: Incomes of workers in an automobile company
Q87: A new surgical procedure is said to
Q101: The learning curve may not be permanent;
Q103: Heidi prepares for an exam by studying
Q105: A salesperson either makes a sale (S)
Q167: If the mean value of a set