What is the standard free-energy change for the following reaction at 25°C? (F = 96,500 C • mol-1) 2Al(s) + 3Sn4+(aq,1 M) → 2Al3+(aq,1 M) + 3Sn2+(aq,1 M) 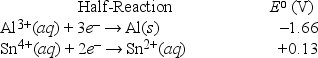
Definitions:
Experiencing Depression
Undergoing or living through the symptoms of depression, characterized by a prolonged period of sadness, hopelessness, or lack of interest in life.
Type A
A personality type characterized by high stress levels, impatience, and a competitive drive, often linked to a higher risk of heart disease.
Type B
Describes individuals who are generally relaxed, less competitive than their Type A counterparts, and more resistant to stress.
Inherited Trait
Refers to characteristics or attributes that are passed from parents to offspring through their genes.
Q5: In the reaction CaO(s) + SO<sub>2</sub>(g) <img
Q7: Consider the reaction in the lead-acid cell
Q29: Which type of radiation is the least
Q41: If the pH of a buffer solution
Q45: What mass of solid CaO is needed
Q55: A 35.0-mL sample of 0.20 M LiOH
Q63: If a captured electron combines with a
Q82: Which of the following type(s) of radiation
Q93: The element oxygen was prepared by Joseph
Q101: _ is used as a subscript beside