Based on the following electrochemical cell, what is the standard reduction potential of metal M at 298 K? (R = 8.314 J/K • mol, F = 96500 C/mol) 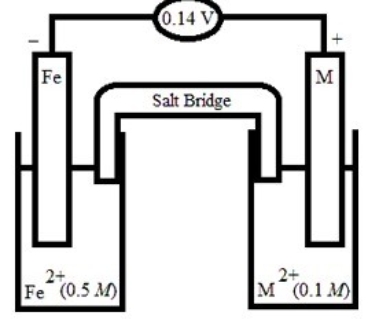
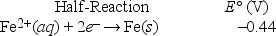
Definitions:
Z Pattern
A movement or visual pattern that follows a zigzag trajectory, often used in eye movement studies or design.
Whisper Test
A simple hearing test in which an examiner whispers a series of words or letters from a set distance to evaluate an individual's hearing ability.
Pinna
The visible part of the ear that is outside the head, which functions to collect and direct sound waves into the ear canal.
Data Recording
The process of collecting and entering data into a recording system or database for analysis, storage, or documentation purposes.
Q22: A voltaic cell is prepared using copper
Q58: The most probable state is the one
Q58: Nuclear fusion is the combination of small
Q65: The Earth's core consists mainly of<br>A) Ni.<br>B)
Q91: What is ΔG°<sub>rxn</sub> for the combustion of
Q92: At a certain temperature the reaction CO<sub>2</sub>(g)
Q93: Which substances are included in the equilibrium
Q116: In order for a gas to be
Q128: Below is a representation of an aqueous
Q131: Which is the appropriate energy-level diagram for