Calculate ΔG° for the combustion of ethanol vapor, C2H5OH(g) , at 750.°C in oxygen to form carbon dioxide and water vapor. The following data are valid at 25°C: 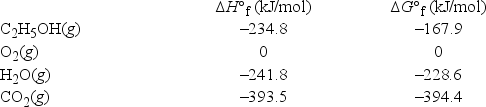
Definitions:
Fluid Intelligence
The ability to use reasoning and solve new problems without relying on knowledge previously gained.
Reorganizing Stage
A phase in various processes (e.g., psychological development, corporate restructuring) where existing structures or strategies are revised and restructured.
Solving Practical Problems
The process of finding solutions to real-world issues or challenges through logical reasoning and practical methods.
Acquiring Knowledge
The process of absorbing, understanding, and retaining new information through various methods such as observation, studies, and experiences.
Q17: Bromothymol blue is a common acid-base indicator.
Q21: Write chemical equations that show what happens
Q32: Reduction occurs at the anode of a
Q41: What is E°<sub>cell</sub> for the following reaction?
Q71: For _-_ reactions K<sub>P</sub> = K<sub>c</sub>(RT)<sup>Δ</sup><sup>n </sup>gas.
Q88: _ occurs at the cathode in a
Q99: Which, if any, of the following processes
Q105: Which statement is correct?<br>A) The cathode is
Q119: The equilibrium constant for the reaction Ni(s)
Q126: What is the name of the molten