For the endothermic reaction A2(g)  2A(g) , a snapshot of an equilibrium mixture of A(g) and A2(g) at low temperature may look as follows. (Each circle represents 1.0 mol of A atoms, and the volume of the box is 1.0 L.)
2A(g) , a snapshot of an equilibrium mixture of A(g) and A2(g) at low temperature may look as follows. (Each circle represents 1.0 mol of A atoms, and the volume of the box is 1.0 L.) 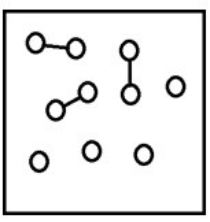 If the system pressure is lowered, what might the new equilibrium system look like?
If the system pressure is lowered, what might the new equilibrium system look like?
Definitions:
Remanufacturing
The process of rebuilding a product to specifications of the original manufactured product using a combination of reused, repaired, and new parts.
Margins
The difference between the cost of goods sold and the selling price, indicating the profit per unit sold.
Emissions
The release of pollutants into the air, including those from industrial processes, transportation, and other sources that can harm human health and the environment.
Production Process
A set of operational activities and tasks involved in the conversion of raw materials into finished products or services.
Q7: Elemental boron can be formed by the
Q18: Find the pH of a 0.20 M
Q37: Which is a statement of the second
Q51: If the adhesive forces between a liquid
Q55: Calcium oxide, CaO, also known as quick
Q64: Discuss modern materials used in medical applications.
Q80: For the endothermic reaction A<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8482/.jpg"
Q90: Which best describes the pH at the
Q114: For the reaction C<sub>6</sub>H<sub>14</sub>(g) → C<sub>6</sub>H<sub>6</sub>(g) +
Q120: Which is the correct equilibrium constant expression