The following is an Arrhenius plot of a first-order reaction. The rate constant is measured in units of s-1. 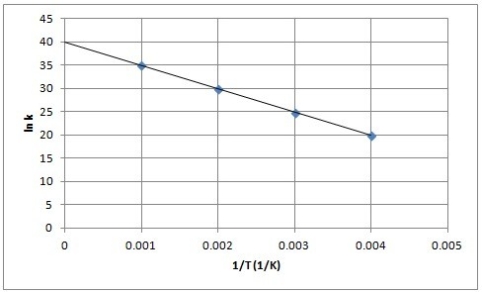 Based on this Arrhenius plot, what is the Arrhenius frequency factor (A) of the reaction? (R = 8.314 J/K mol)
Based on this Arrhenius plot, what is the Arrhenius frequency factor (A) of the reaction? (R = 8.314 J/K mol)
Definitions:
Profit-Maximizing
The strategy or method of modifying the production and sales of products and services to attain the maximum possible profit.
Monopolist
A singular entity or company that has exclusive control over the supply of a particular good or service, giving it significant market power.
P > MR
This inequality indicates a scenario in market pricing where the price (P) of a good exceeds its marginal revenue (MR), common in imperfectly competitive markets.
Positive Economic Profits
Occurs when the total revenues of a firm exceed the total costs, including opportunity costs.
Q7: Which of the following salts will form
Q7: What is meant by HIPS?<br>A) High polysaccharides<br>B)
Q16: There are guidelines to help write equilibrium
Q20: Which of the following sets of conditions
Q45: The rate law for the reaction H<sub>2</sub>O<sub>2</sub>
Q60: Hydrogen sulfide can be formed in the
Q72: What is the pH of a 0.050
Q98: Which law describes the quantitative relationship between
Q101: Octane has a vapor pressure of 40.
Q121: Helium atoms do not combine to form