Solid sodium peroxide (Na2O2) reacts with liquid water yielding aqueous sodium hydroxide and oxygen gas. How much heat is released if 327.2 g of oxygen gas is produced from the reaction of sodium peroxide and water under standard-state conditions? 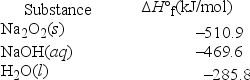
Definitions:
Pediatrician
A medical doctor specializing in the care and treatment of children's diseases and health issues.
American Health Information Management Association
A professional organization dedicated to improving health record quality and promoting the effective management of personal health information.
Clinical Medical Assistant
A healthcare professional who performs administrative and clinical tasks in support of physicians and other health practitioners, primarily in outpatient or ambulatory care facilities.
American Hospital Association
A professional organization that promotes the interests and standards of hospitals and health care networks in the United States.
Q4: Once the following equation is balanced with
Q13: How many electrons are in the 4p
Q14: The empirical formula is the simplest whole
Q24: What is the oxidation number of sulfur
Q30: Based on the following thermochemical equations, what
Q30: Balance the following oxidation-reduction equation: Li(s) +
Q70: Which one of the following substances is
Q81: What is ΔH°<sub>rxn</sub> for the decomposition of
Q81: Select the compound with the lowest (i.e.,
Q119: How many total electrons are present in