TABLE 14-18
A logistic regression model was estimated in order to predict the probability that a randomly chosen university or college would be a private university using information on mean total Scholastic Aptitude Test score (SAT) at the university or college, the room and board expense measured in thousands of dollars (Room/Brd), and whether the TOEFL criterion is at least 550 (Toefl550 = 1 if yes, 0 otherwise.) The dependent variable, Y, is school type (Type = 1 if private and 0 otherwise).
The Minitab output is given below: 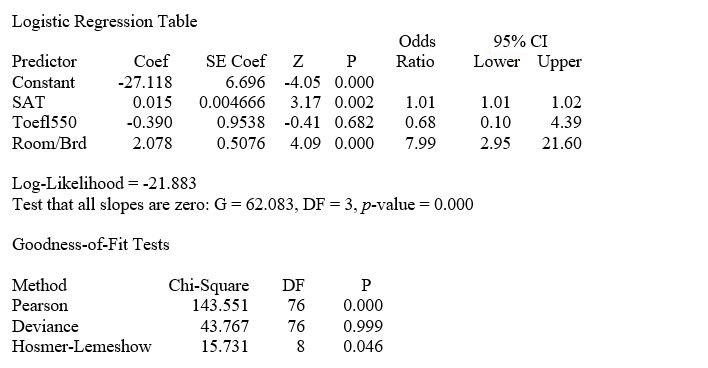
-Referring to Table 14-18, what is the p-value of the test statistic when testing whether Toefl500 makes a significant contribution to the model in the presence of the other independent variables?
Definitions:
H2O
The chemical formula for water, a compound consisting of two hydrogen atoms bonded to one oxygen atom.
Balanced Equation
A chemical equation where the number of atoms for each element is equal on both the reactant and product sides, indicating mass conservation.
SiO2
The chemical formula for silicon dioxide, a naturally occurring oxide of silicon commonly found in quartz and many other minerals.
Balanced Equation
A chemical equation that has equal numbers of atoms for each element involved in the reaction on both reactant and product sides, adhering to the law of conservation of mass.
Q4: In the United States, the control limits
Q5: Every spring semester, the School of Business
Q7: In multiple regression, the _ procedure permits
Q29: A Paso Robles wine producer wanted to
Q31: To assess the adequacy of a forecasting
Q37: Referring to Table 14-4, which of the
Q53: One of the morals of the red
Q85: Referring to Table 13-3, the director of
Q106: Referring to Table 16-4, a centered 3-year
Q231: Referring to Table 14-15, the null hypothesis