Why is 2,4,5-trifluorophenol much more acidic than phenol? 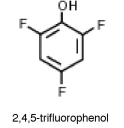
Definitions:
Consciousness Raising
The process of increasing awareness about social, political, or personal issues or inequities, often with the goal of inducing change.
Information Reprocessing
A cognitive approach aimed at helping individuals reframe and understand information in a way that leads to emotional and psychological healing.
Traumatic Memories
Memories of events that are emotionally disturbing or distressing, which can negatively impact an individual's mental health.
Traditional Behavioral Model
The traditional behavioral model is a psychological framework that emphasizes observable and measurable behaviors, focusing on the learning processes that lead to behavior change.
Q3: Two species of frogs do not interbreed
Q18: The work of _ finally convinced the
Q28: Metamorphism caused by the close proximity of
Q108: When blue food coloring is stirred into
Q127: Why does an atom with many valence
Q141: The elements that make the best reducing
Q151: The boiling point of 1,4-butanediol is 230°C.
Q175: Which of the following solutions is the
Q180: Bond energies increase in going from C-N
Q228: Which of the following statements describes a