Definitions:
Standard Gibbs Free Energy
A thermodynamic property used to predict the direction of chemical reactions and the extent to which reactions proceed.
Enthalpy
A thermodynamic property representing the total heat content of a system, used to quantify the heat changes that occur in chemical reactions at constant pressure.
Entropy
A measure of the disorder or randomness in a system, often related to the number of available states a system can occupy.
% Conversion
The percentage indicating how much of the reactants have been transformed into products in a chemical reaction.
Q107: The table shows a schedule of
Q142: Describe two basic differences between the mainstream
Q166: One reason the lowest wage rate is
Q228: The World Trade Organization was established by
Q232: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8601/.jpg" alt=" In the curve,
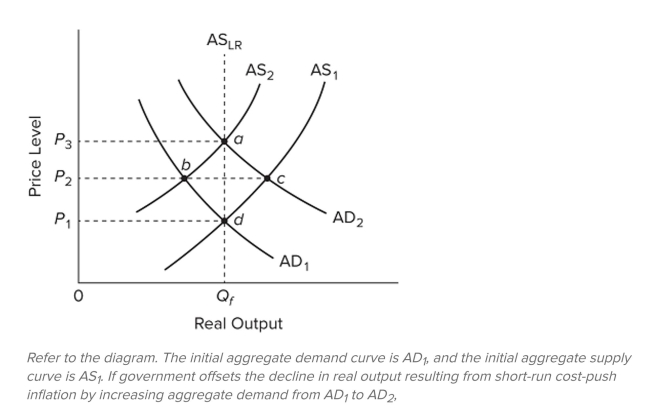
Q238: The short-run aggregate supply curve illustrates the
Q241: Monetarists argue that government policy interference in
Q242: Tariffs and import quotas meant to increase
Q267: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8601/.jpg" alt=" A)
Q323: Risk in finance means<br>A) mostly positive outcomes.<br>B)