What is the cell potential (E0) for a galvanic cell formed from the following two half-reactions? Assume that the cell temperature is 45°C and the operating pressure is 0.06 atm. 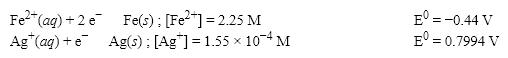
Definitions:
Cellular Respiration
The metabolic process by which cells produce energy in the form of ATP from glucose.
Desert Organisms
Plants and animals specifically adapted to survive in harsh desert environments.
Citric Acid Cycle
A series of chemical reactions used by all aerobic organisms to release stored energy through the oxidation of acetyl-CoA derived from carbohydrates, fats, and proteins into carbon dioxide and chemical energy.
Electron Transport Chain
A series of protein complexes embedded in the mitochondrial or cellular membrane that facilitates the transfer of electrons, generating a proton gradient used to produce ATP.
Q4: Directions: Choose the best answer based on
Q7: Directions: Identify the agreement error in the
Q8: Limestone (CaCO<sub>3</sub>)emits CO<sub>2</sub>gas when exposed to HCl
Q10: _ suggested that electrons occupy stable orbits
Q10: Directions: Apply the knowledge you have gained
Q12: Directions: Choose the best answer based on
Q13: The following reaction represents the complete combustion
Q14: Electron capture leads to the:<br>A)emission of an
Q15: A coordinating conjunction joins clauses that are
Q44: Which of the following isotopes has the