When 10.0 g of ethane, C₂ H₆(g) , burns in oxygen, 519 kJ of heat are released. What is D H for the following thermochemical equation?
2 C₂ H₆(g) + 7 O₂(g) 4 CO₂ (g) + 6 H₂ O ( 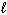 )
)
Definitions:
Across Cultures
A term referring to the comparison or interaction between different cultures or cultural practices, emphasizing diversity and multicultural understanding.
Principled Negotiation
A negotiation strategy focused on mutual interests rather than positions, aimed at finding a solution that is fair to all parties involved.
Negotiating Parties
Individuals or groups engaged in discussions to reach a mutual agreement.
Negotiators
Individuals who engage in dialogue with the intent to reach an agreement or compromise on a matter of mutual interest.
Q1: How many moles of argon gas are
Q12: What is the concentration of a H<sub>2</sub>SO<sub>4</sub>
Q16: Given the data below, what approximately is
Q16: One type of sunburn occurs on exposure
Q33: Which element would be expected to have
Q37: Exhibit 4-2 Consider mixing an aqueous solution
Q66: Arrange the elements N, O and Si
Q67: Which element has the valence electrons 4s<sup>2</sup>3d<sup>2</sup>?<br>A)
Q69: What was the result of the Rutherford
Q143: What is the mass of hydrogen in