Given the data below, what is D H for the following reaction?
4 NO (g) + 6 H₂ O ( 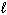 ) 4 NH₃(g) + 5 O₂(g) D H f H₂ O (
) 4 NH₃(g) + 5 O₂(g) D H f H₂ O ( 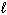 ) = - 286 kJ/mol D H f NH₃(g) = - 46 kJ/mol D H f NO (g) = 90 kJ/mol
) = - 286 kJ/mol D H f NH₃(g) = - 46 kJ/mol D H f NO (g) = 90 kJ/mol
Definitions:
4-methylpentanal
An organic compound, specifically an aldehyde, with the chemical formula C6H12O, characterized by a methyl group attached to the fifth carbon of a pentanal chain.
Sodium Hydroxide
A highly caustic and strong base used in many industries; it is also known as lye or caustic soda.
α,β-unsaturated Aldehyde
An organic compound with a double bond between the alpha and beta carbon atoms and a carbonyl group at the alpha position.
β-diketone
A class of organic compounds containing two keto groups (C=O) attached to adjacent carbon atoms.
Q18: What is the order for filling the
Q23: Which of the following bonds would be
Q65: The energy of the electron in the
Q69: How much heat must be added to
Q78: Exhibit 8-1 The following question(s) relate to
Q80: What is the definition for Molarity ?<br>A)
Q106: What is the density of SO<sub>2</sub> gas
Q113: Consider the reaction between hydrazine, N<sub>2</sub>H<sub>4</sub>, and
Q113: The nucleus of an atom contains what
Q121: The element sulfur forms compounds that contain