Given the following thermochemical equations and their corresponding enthalpies:
2 C₂ H₂ (g) + 5 O₂(g) 4 CO₂ (g) + 2 H₂ O ( 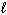 ) D H = - 2601 kJ C8 H8 (
) D H = - 2601 kJ C8 H8 ( 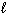 ) + 10 O₂(g) 8 CO₂ (g) + 4 H₂ O (
) + 10 O₂(g) 8 CO₂ (g) + 4 H₂ O ( 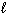 ) D H = - 4393 kJ What is the enthalpy change for the following reaction?
) D H = - 4393 kJ What is the enthalpy change for the following reaction?
4 C₂ H₂ (g) C8 H8 ( 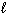 ) D H = ?
) D H = ?
Definitions:
SPSS Modeler
A software package used for statistical analysis and building predictive models.
Data Mining
The process of discovering patterns and knowledge from large amounts of data. The data sources can include databases, data warehouses, the Internet, and other data repositories.
Pivot Tables
A feature in spreadsheet software that allows users to reorganize and summarize selected columns and rows of data in a spreadsheet.
SPSS Syntax
A programming language used in the SPSS software for performing data analysis, manipulation, and visualization.
Q2: What is the specific heat of an
Q25: Which species shown below is largest?<br>A) In<br>B)
Q36: Which of the following defines the heat
Q46: What is the molarity of a solution
Q56: In which of the following molecules does
Q58: Which molecule listed below has more than
Q74: Given the following reaction and its corresponding
Q75: What is the mass percent of calcium
Q91: What is the steric number with respect
Q133: What bonded atom lone pair arrangement is