Given the standard heats of formation listed below, what is D H for the following reaction?
C6 H12 O6 (s) + 6 O₂(g) 6 CO₂ (g) + 6 H₂ O ( 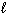 ) D H f C6 H12 O6 (s) = - 1268 kJ/mol D H f CO₂ (g) = - 394 kJ/mol D H f H₂ O (
) D H f C6 H12 O6 (s) = - 1268 kJ/mol D H f CO₂ (g) = - 394 kJ/mol D H f H₂ O ( 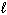 ) = - 286 kJ/mol
) = - 286 kJ/mol
Definitions:
Drawings
Visual representations that detail the specifications of objects, structures, or systems.
Planning And Control
The process of strategic decision making and operational oversight to manage an organization's resources efficiently and effectively to achieve desired outcomes.
Empowered Individuals
People who have been given authority and responsibility, enabling them to take action and make decisions independently.
Common Goal
A shared objective among a group of individuals or within an organization, fostering unity and collaborative efforts.
Q4: What is the volume in milliliters of
Q10: Given the three statements below, pick the
Q26: Calculate the pressure of 1.33 mol of
Q43: Which Lewis structure shown below for BeCl<sub>2</sub>
Q48: Which atom from the list below normally
Q62: Which describes a set of elements on
Q77: Under which of the following conditions would
Q112: Which atom from the list below normally
Q117: Refer to Exhibit 6-1. What is the
Q164: A 1.037 g sample of a substance