For which of the equations shown below does the standard enthalpy of reaction equal the standard molar enthalpy of reaction of the product?
I. 1 / 2 N₂(g) + 3 / 2 H₂ (g) NH₃(g)
II. H₂ (g) + Br₂( 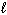 ) 2 HBr (g)
) 2 HBr (g)
III. HCl (g) + NH₃(g) NH ₄Cl (s)
Definitions:
Endocrine System
A network of glands and organs that produce, store, and secrete hormones into the bloodstream to regulate the body’s growth, metabolism, and function.
Neuromodulators
Chemicals released by nerve cells to signal, modify, and regulate the activity of other neurons.
Synaptic Transmission
The process by which one neuron communicates with another by passing chemical or electrical signals across a synapse.
Neurotransmitter Receptor
A protein molecule on a neuron's surface that reacts to a specific neurotransmitter, influencing neural communication.
Q1: Three possibly correct resonance forms of N₂O<sub>4</sub>
Q14: The molecule H<sub>2</sub>O<sub>2</sub> (H - O -
Q37: Given the three Lewis structures listed below,
Q44: How many valence electrons does a silicon
Q46: Arrange the following elements in order of
Q56: The species containing 6 protons and 5
Q69: The total pressure exerted by a mixture
Q75: Refer to Exhibit 7-1. Which of the
Q105: Pick the correct Lewis structure shown below.<br>A)
Q147: Complete and balance the following acid-base neutralization