Given the three Lewis structures listed below, pick the best answer.
I. 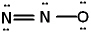
II. 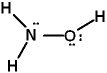
III. 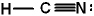
Definitions:
Oxide Minerals
Minerals composed of oxygen and one or more metals, common in the Earth's crust and important in various geological processes.
Calcite
A common mineral form of calcium carbonate, CaCO3, often found in sedimentary rocks such as limestone.
Covalent Bonds
Chemical bonds formed by the sharing of electron pairs between atoms.
Intermolecular Bonds
Forces of attraction or repulsion between molecules, crucial for determining the physical properties of substances.
Q13: Which of the following molecules is(are) polar
Q14: A 3.60 m solution of KBr (molar
Q32: In which solvent would the solubility of
Q35: The element with a ground state electron
Q37: Given the equation below, what is D
Q65: The ground state electron configuration of Ca
Q83: In the titration of a 50.0 mL
Q87: Calcium fluoride, CaF<sub>2</sub>, has a unit cell
Q96: What is the formal charge of the
Q100: Which of the following forces between atoms