What is the enthalpy of reaction D H rxn for the following reaction?
SiO₂ (s) + 4 HF (g) SiF4 (g) + 2 H₂ O ( 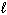 ) D H rxn = ??
) D H rxn = ??
D H f (SiO₂ ) = - 910.9 kJ/mol D H f (HF) = - 273.0 kJ/mol D H f (SiF4 ) = - 1614.9 kJ/mol D H f (H₂ O) = - 285.8 kJ/mol
Definitions:
Good Deal
A situation or agreement in which a person or party receives great value or advantage, often in terms of quality, price, or terms of sale.
New Boat
A term referring to a newly manufactured or newly acquired boat, fresh from production or purchase.
Weak Closing Statements
Inadequate or ineffective statements made at the end of a presentation or sales pitch, failing to strongly persuade or encourage a decision.
Successful Sales Call
A sales interaction that achieves its intended outcomes, such as progressing a sale, creating a positive relationship, or closing a deal.
Q19: The element with a ground state electron
Q23: When a 40.0 g sample of a
Q42: For which of the equations shown below
Q67: The quantum number <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBX8714/.jpg" alt="The quantum
Q83: What are the molar volume, temperature and
Q89: Which species is not isoelectronic with the
Q95: What is the molar mass of magnesium
Q101: The H - X - H angle
Q105: Pick the correct Lewis structure shown below.<br>A)
Q182: What is the mass of 12.04×10<sup>23</sup> atoms