Given the data below, what is D H for the following reaction?
2 CH4 (g) + 3 O₂(g) 2 CO (g) + 4 H₂ O ( 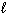 ) D H f CH4 (g) = - 75 kJ/mol D H f CO (g) = - 110 kJ/mol D H f H₂ O (
) D H f CH4 (g) = - 75 kJ/mol D H f CO (g) = - 110 kJ/mol D H f H₂ O (  ) = - 286 kJ/mol
) = - 286 kJ/mol
Definitions:
Profitability Analysis
An assessment process to determine the ability of a business to generate earnings as compared to its expenses and other relevant costs incurred during a specific period.
Net Income
The total profit of a company after all expenses, including taxes and costs, have been subtracted from total revenue.
Solvency
The ability of a company to meet its long-term financial obligations and continue its operations in the long term.
Accumulated Other Comprehensive Income
The cumulative effect of other comprehensive income items, which is reported separately in the “Stockholders’ equity” section of the balance sheet.
Q2: The systematic name of the compound H<sub>2</sub>S
Q20: What is the volume in liters of
Q20: Consider the polyatomic ion, ClO<sup>1 - </sup>.
Q47: In the reaction of 5.44 g of
Q56: How many unpaired electrons does the ground
Q73: What volume of 1.1 M HCl is
Q79: The bonded atom lone pair arrangement about
Q83: The Lewis structure for formaldehyde (CH<sub>2</sub>O) is:<br>A)
Q97: How many grams of N<sub>2</sub>H<sub>4</sub> are produced
Q165: How many oxygen atoms are present in