What is the standard molar enthalpy change D H rxn for the following reactions using the standard molar enthalpies of formations provided?
SiCl 4 ( 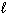 ) + 2 H₂ O (
) + 2 H₂ O ( 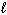 ) SiO₂ (s) + 4 HCl (g) D H rxn = ???
) SiO₂ (s) + 4 HCl (g) D H rxn = ???
D H f (SiCl 4 ) = - 640.1 kJ/mol D H f (SiO₂ ) = - 910.9 kJ/mol D H f (H₂ O) = - 285.8 kJ/mol D H f (HCl) = - 92.3 kJ/mol
Definitions:
Counseling Efficacy
The effectiveness or success of counseling interventions or practices.
Control Group
In experimental research, a group of participants that does not receive the treatment or intervention, serving as a baseline to compare the effects of the treatment.
Dismantling Strategy
An approach in research or therapy that involves breaking down a complex intervention into its components to understand its effectiveness.
Additive Strategy
In problem-solving, an approach where solutions are built by sequentially adding components that contribute toward a solution.
Q1: A chemist places (NH<sub>4</sub>)<sub>2</sub>SO<sub>4</sub> in one flask
Q1: Which species listed below has 15 protons,
Q6: Which element has the valence electrons 3s<sup>2</sup>3p<sup>2</sup>?<br>A)
Q19: What mass of AgCl is produced by
Q45: Given the three statements below, pick the
Q54: What is the mass of hydrogen in
Q60: Pick the one statement listed below that
Q66: Liquid hydrogen peroxide is an oxidizing agent
Q112: Which subatomic particles listed below are considered
Q145: In the reaction oF<sub>4</sub>4.5 g of gallium