Consider the following first order reaction for the decomposition of dinitrogen pentoxide:
N₂O5(g) 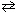 2 NO₂ (g) + O₂(g) At 70 C, the rate constant, k , for this reaction equals 6.82 * 10-3 s-1 . If the initial concentration of N₂O5 equals 0.0125 M, how long would it take for the concentration of N₂O5 to reduce to 0.00500 M?
2 NO₂ (g) + O₂(g) At 70 C, the rate constant, k , for this reaction equals 6.82 * 10-3 s-1 . If the initial concentration of N₂O5 equals 0.0125 M, how long would it take for the concentration of N₂O5 to reduce to 0.00500 M?
Definitions:
Right Ventricle
The lower right chamber of the heart that pumps oxygen-depleted blood to the lungs for oxygenation.
Pulmonary Trunk
A major blood vessel that carries deoxygenated blood from the right ventricle of the heart to the lungs for oxygenation.
Cushion the Heart
Likely refers to the protective layer around the heart, such as the pericardial fat or the pericardium, but without specific context, the term is ambiguous.
Outermost Layer
The external surface or coating of an object or body.
Q14: What is the pH of an aqueous
Q42: Given:<br>2 Cl₂ (g) + 2 H₂ O
Q42: Consider the following unfinished table of weak
Q44: Refer to Exhibit 13-16. What is the
Q56: Which experiment listed below would provide a
Q59: What is the equilibrium constant expression for
Q65: Refer to Exhibit 15-4. What is the
Q77: Benzene boils at 80.10 ° C and
Q78: A weak monoprotic acid is dissolved in
Q207: At high temperature, the following gas phase