The half-life of the first order radioactive decay of 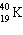 is 1.30 10 9 years. How long would it take for the
is 1.30 10 9 years. How long would it take for the 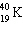 to decay to 25% of its original concentration?
to decay to 25% of its original concentration?
Definitions:
Predetermined Overhead Rate
A rate used to allocate manufacturing overhead costs to individual products or job orders, based on a formula that estimates the costs.
Machine-Hours
Machine-hours refer to the total time that machinery is operated within a specific period, used as a basis for allocating manufacturing overhead to products.
Variable Manufacturing Overhead
The part of manufacturing overhead expenses that directly fluctuates in relation to the volume of production.
Machine-Hours
A measurement of the amount of time a machine is operated, used as a basis for allocating machine-related costs to products.
Q18: What is the bond order for the
Q24: What is the concentration of Pb 2+
Q37: The gas phase reaction C<sub>2</sub>H<sub>4</sub> + Cl<sub>2</sub>→C<sub>2</sub>H<sub>4</sub>Cl<sub>2</sub>
Q41: Measurements show that the [OH<sup> - </sup>]
Q65: The equilibrium governed by the reaction 4
Q72: The K <sub>sp</sub> for PbBr<sub>2</sub> is 4.0×10<sup>
Q74: At 25 C, K p = 0.240
Q78: A weak monoprotic acid is dissolved in
Q102: Glacial acetic acid, HC<sub>2</sub>H<sub>3</sub>O<sub>2</sub>, is 99.8% (m/m).
Q122: What type of hybrid orbitals are used