The equilibrium constant for the reaction below is 2.5*109 at a particular temperature.
2 SO₂ (g) + O₂(g) 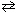 2 SO3(g)
2 SO3(g)
What can be stated about the relative equilibrium distribution for this mixture?
Definitions:
Impressions
The initial perceptions or thoughts formed about someone or something based on appearance, behavior, or acquired information.
Thinking
The process of using one's mind to consider or reason about something, often leading to problem-solving or decision-making.
Encoding
The process by which information is transformed into a format that can be stored in memory.
Assigned
Allocated or designated to a person, task, or responsibility by someone in authority.
Q1: When 20.0 grams of a water soluble
Q19: What is the transition from the gaseous
Q22: The number of p bonds present in
Q30: A physician studying a mutant, hemoglobin, blood
Q60: For the reaction 2 SO₂ (g) +
Q103: What bonded-atom lone-pair arrangement is associated with
Q140: Refer to Exhibit 13-8. What is the
Q143: Consider the conversion of oxygen, O<sub>2</sub>, to
Q163: Arrange the following solutions in order of
Q211: What is the concentration of F<sup> -