Multiple Choice
Given:
N₂(g) + 3 H₂ (g) 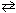 2 NH₃K c (eq 1) = 2.25*10- 6 What is the equilibrium-constant, K c , for the formation of one mole of ammonia from nitrogen and hydrogen at 600 K as shown below?
2 NH₃K c (eq 1) = 2.25*10- 6 What is the equilibrium-constant, K c , for the formation of one mole of ammonia from nitrogen and hydrogen at 600 K as shown below?
1 / 2 N₂(g) + 3 / 2 H₂ (g) 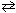 NH₃K c (eq 2) = ??
NH₃K c (eq 2) = ??
Definitions:
Related Questions
Q33: Which of the following would have an
Q59: What is the equilibrium constant expression for
Q62: K c = 0.35 for the reaction:<br>H₂
Q64: Rank the following three acids in terms
Q127: Which set(s) of materials listed below will
Q138: Under what conditions does changing the pressure
Q142: At 1000 ° C, cyclobutane (C<sub>4</sub>H<sub>8</sub>) decomposes
Q149: Sulfur trioxide decomposes at high temperature in
Q164: Some N₂H<sub>4</sub> is placed in a 1.0
Q186: A solution is made by mixing 50