Some N₂H4 is placed in a 1.0 L container, which is sealed, and raised to 1273 K. After the reaction, chemical analysis shows that the concentration of H₂ is 0.20 M , N₂is 0.10 M , and N₂H4 is 0.10 M . Calculate K c for:
N₂H4 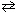 N₂+ 2 H₂
N₂+ 2 H₂
Definitions:
Allowance Account
An account used in accounting to record reductions in the carrying amount of accounts receivable, due to potential or estimated uncollectible debts.
Net Realizable Value
The estimated selling price in the ordinary course of business minus the estimated costs of completion and the estimated costs necessary to make the sale.
Accumulated Depreciation
The total amount of depreciation expense that has been recorded for an asset since it was acquired.
Fair Value
The estimated market value of an asset or liability, reflecting what a willing buyer would pay a willing seller in an arm's length transaction.
Q13: Which substance(s) listed below has(have) a standard
Q36: Refer to Exhibit 13-7. What is the
Q45: A 25.0 mL volume of a 0.200
Q49: Consider solving for the molar solubility of
Q64: When the reversible reaction, N₂+ O₂ <img
Q86: Pyridine (p K <sub>b</sub> = 8.74) reacts
Q94: Refer to Exhibit 13-3. What is the
Q100: Which of the following forces between atoms
Q126: In the acid-base equilibrium PO<sub>4</sub> 3 -
Q162: An equilibrium mixture at 500 K has